Have you ever wondered why some materials conduct electricity while others don’t? In today’s blog post, we’ll explore the fascinating world of conductors and insulators. We’ll give you a simple definition of what these terms mean and explore their significance in our daily lives.
From copper wires to rubber gloves, conductors and insulators play a vital role in various electrical and thermal applications. Understanding the characteristics of different materials can help us make informed decisions, whether it’s choosing the right wiring for our homes or selecting the most suitable materials for electrical devices. So, let’s dive into the intriguing world of conductors and insulators and discover their properties through real-life examples.
So, what is the difference between a conductor and an insulator? Why do some materials allow electricity or heat to flow through them while others block it? Join us as we unravel the mysteries of conductors and insulators, providing you with concrete examples along the way.
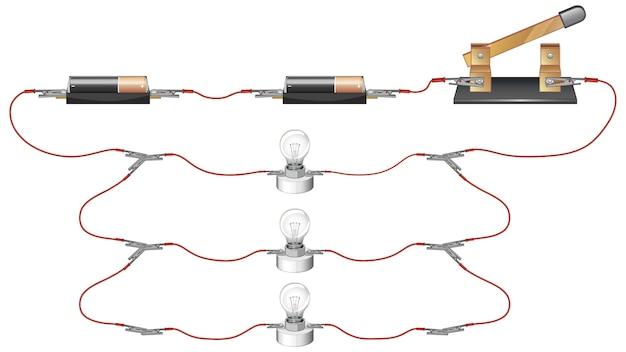
What are Conductors and Insulators? Give One Example Each
Conductors and insulators are terms commonly used in the field of physics to describe the ability of materials to conduct or resist the flow of electric current. While they may sound like characters straight out of a superhero movie, they play a crucial role in our everyday lives, shaping the way electricity behaves around us. Let’s dive into their definitions and explore one example for each to shed some light on this electrifying subject!
Conductors: Channeling Electricity Like a Pro
Conductors are materials that allow the easy flow of electric current. Just imagine them as the MVPs (Most Valuable Players) of the electrical world, effortlessly guiding the flow of electricity with their exceptional conducting abilities. These materials possess a large number of free electrons that are not tightly bound to their atoms, making it easier for electric current to flow through them.
Example: Copper – The Electrical Powerhouse
When it comes to conducting electricity, copper takes the crown! Its high electrical conductivity and abundance in nature have made it an ideal choice for wiring in countless electrical applications. From power lines delivering electricity across cities to the delicate circuitry inside electronic devices, copper wires are the go-to conductor for the job. So, next time you flip the switch and your lights turn on, give a nod of appreciation to our friendly neighborhood copper!
Insulators: The Electricity Defenders
On the opposite side of the spectrum, we have insulators. These materials, aptly named, are like the guardians of electricity, preventing its flow and keeping it in check. Insulators have tightly bound electrons that do not move freely, which hinders the flow of electric current through them. It’s like they’ve built an impenetrable fortress around the electricity, shielding it from venturing into places it shouldn’t be.
Example: Rubber – the Electrically-Sturdy Shield
Meet rubber, the unsung hero protecting us from the perils of electrical conductivity! Its low conductivity and excellent insulation properties make it an invaluable component for electrical wires and cables. The rubber insulation ensures that the electric current stays contained within the wires, reducing the risk of shock or short-circuits. So, the next time you unplug a device while holding onto the rubber plug, remember to thank this trusty insulator for protecting you from any shocking encounters!
In a world filled with the wonders of electricity, conductors and insulators take center stage, playing their remarkable roles in ensuring our safety and enabling the flow of power. Whether it’s the mighty copper conducting the charge or the durable rubber shielding us from its path, these materials make electricity dance to their tunes. So, let’s embrace their electrifying performances and appreciate the science that keeps our world buzzing!
Note: The information in this blog post is for educational purposes only and is not intended as a comprehensive guide. Always exercise caution and consult professionals for specific electrical concerns or inquiries.
Conductor and Insulator: A Comprehensive FAQ
Welcome to our comprehensive FAQ on conductors and insulators! If you’ve ever been curious about what these terms mean and how they relate to the world around us, you’ve come to the right place. In this article, we’ll provide simple definitions, examples, and answer some burning questions you may have had.
What is the Simple Definition of an Insulator
An insulator is a material that does not allow electricity to flow through it easily. In other words, it hinders the movement of electric charge. Insulators have high electrical resistance, which makes them useful for providing insulation and protection from electrical current. Think of them as electric traffic cops—keeping the charge from going where it’s not supposed to!
What are Three Examples of Conductors and Three Examples of Insulators
Conductors are materials that easily allow the flow of electric charge. They are the “good guys” when it comes to conducting electricity. Here are three examples of conductors:
-
Copper: Copper is a superstar conductor that’s widely used in electrical wiring. It has excellent conductivity and is used in everything from power lines to electronic devices.
-
Aluminum: Another popular conductor, aluminum is commonly found in power grids, light bulbs, and even smartphone chargers.
-
Silver: Known for its high electrical conductivity, silver is used in various applications such as circuit boards and high-performance audio cables.
While conductors are essential, insulators play an equally important role. Here are three examples of insulators:
-
Rubber: Rubber is commonly used to insulate electrical wires, providing protection against electric shock. Its high resistance to electric flow makes it an excellent insulator.
-
Glass: Remember when your parents warned you about mixing electricity and water? Well, glass is one of the materials that helps keep us safe. It’s a great insulator due to its high resistance.
-
Plastic: From electric plugs to circuit boards, plastic is commonly used as an insulator in electronic devices. Its low conductivity keeps us far away from those pesky electric shocks.
Can Solid NaCl Conduct Electricity
As much as we all love salt, solid sodium chloride (NaCl) is not a conductor of electricity. It’s actually an example of an insulator because ionic compounds, like NaCl, do not allow the flow of electricity in their solid state. However, when dissolved in water, NaCl breaks down into ions, allowing it to conduct electricity as an electrolyte solution.
What is the Poorest Conductor of Electricity
If there were awards for being a lousy conductor, the carbon compound known as graphite would take home the trophy. Despite being primarily made up of carbon, which can conduct electricity as diamonds do, graphite consists of layers that don’t hold onto electrons tightly. As a result, it has a significantly higher electrical resistance compared to metals or even other forms of carbon.
Is Magnesium a Good Conductor of Electricity
Now, this is where things get interesting! Magnesium is indeed a conductor of electricity, but it’s not the best out there. Compared to copper or aluminum, magnesium’s electrical conductivity falls a bit short. However, it still conducts well enough to be used in various electrical applications, such as car wiring and electronic devices.
Is Calcium a Good Insulator
Although calcium is a vital element for our bones and overall health, it doesn’t make the cut as an insulator. Calcium is actually a metal and, like most metals, it exhibits good conductivity. So, if you’re looking for an insulator, keep your calcium supplements away from electrical circuits!
Can C6H12O6 Conduct Electricity
Ah, the sweet nectar of life—glucose! Chemical formula C6H12O6. However, when it comes to conducting electricity, glucose falls into the insulator category. This organic compound doesn’t conduct electricity in either its solid or aqueous state. So, while it might provide a burst of energy for our bodies, it won’t be giving any electrical charge a boost.
Is Na2SO4 a Good Conductor of Electricity
If you’re exploring the world of ionic compounds, Na2SO4 (sodium sulfate) is an interesting one. When in its solid state, Na2SO4 is not a conductor of electricity. However, when dissolved in water or melted, it dissociates into sodium (Na+) and sulfate (SO4²-) ions, thereby becoming an electrolyte solution capable of conducting electricity.
Is Vinegar a Good Conductor of Electricity
Time to bring out the pickles and highlight another interesting example! When it comes to vinegar—an acid solution mainly composed of acetic acid (CH3COOH)—it does have some conductivity. However, vinegar’s conductivity is relatively low compared to more potent electrolyte solutions. So, while it may add flavor to our salads, it certainly won’t electrify your circuits.
Does Calcium Conduct Heat
Indeed, calcium does conduct more than just electricity! As a metal, calcium also acts as a good conductor of heat. So, if you’re cooking up something delicious and need even heat distribution, calcium might be lending a helping hand in your cookware!
Does Calcium Conduct Electricity in Liquid State
While calcium can conduct electricity as a solid, unfortunately, it loses its conductivity when melted or in a liquid state. So, you won’t find liquid calcium secretly running any electrical currents. Instead, it will happily simmer away, leaving the electrical charge for other conductors to handle.
We hope this FAQ section has shed some light on conductors and insulators, providing you with a comprehensive understanding of their definitions and examples. From copper wiring to rubber insulation, from magnesium conductivity to vinegar’s electrolytic secrets, these concepts play a fascinating role in our everyday lives. So, next time you flick on a light switch or handle an electrical device, take a moment to appreciate the incredible science happening right before your eyes—conduction and insulation at work, paving the way for modern technology!
