Enzymes, the microscopic powerhouses of our body’s biochemical reactions, play a crucial role in maintaining the delicate balance of life. But have you ever wondered how the pH level can affect their activity? Join me on a fascinating journey as we dive into the experiments exploring the relationship between pH and enzyme activity.
In this blog post, we will demystify the effect of pH on enzyme activity, answering questions like: What happens when enzymes are not in their optimal pH? At what pH is an enzyme most effective? And why is the optimum pH of amylase 7? Along the way, we will understand the significance of pH and its impact on enzyme kinetics. So, grab your lab coat and let’s uncover the secrets of how pH influences enzyme activity in this captivating scientific exploration.
Keywords: How do you kill enzymes?, How does pH affect enzyme activity experiment?, Why is the optimum pH of amylase 7?, What is the optimum pH of amylase?, At what pH would the enzyme be most effective?, How does adding more substrate affect an enzyme reaction?, What happens if an enzyme is inhibited?, What happens when enzymes are not in their optimal pH?
How pH Can Add a Zing to Enzyme Activity Experiments
Picture this: a bunch of enzymes hanging out in a test tube, ready to party. But what’s a party without the right pH? That’s right, pH can have a major impact on enzyme activity. So let’s dive into the electrifying world of pH and enzyme experiments, shall we?
Unraveling the pH Puzzle
pH, for the uninitiated, stands for “power of hydrogen.” It’s a measure of acidity or alkalinity, and it can range from 0 to 14. A pH of 7 is neutral, below 7 is acidic, and above 7 is alkaline. Enzymes, on the other hand, are like the superheroes of the biochemical world. They speed up chemical reactions, making them happen faster than a speeding bullet. But like any superhero, enzymes have their kryptonite, and in this case, it’s pH.
The pH Party Crashers
When it comes to enzyme activity, pH can make or break the party. Enzymes have a sweet spot, a pH at which they perform their best. Stray too far from this pH and they start losing their superpowers. It’s like trying to dance ballet in high heels – sure, it’s possible, but it’s not going to be pretty.
Throwing Acid and Alkali into the Mix
To observe how pH affects enzyme activity, scientists whip out their lab coats and get experimental. They set up a series of test tubes, each containing the same enzyme but with different pH levels. Then, they add a substrate – the molecule the enzyme acts upon, like a secret dance partner.
Busting Out the pH Scale
The first test tube may have a pH of 2, about as acidic as a lemon. The next one could be around pH 7, a cool and neutral companion. And the last one? Let’s crank it up to pH 12 like a strong alkaline solution. Then, they measure the enzyme’s activity in each tube, observing how fast it breaks down the substrate.
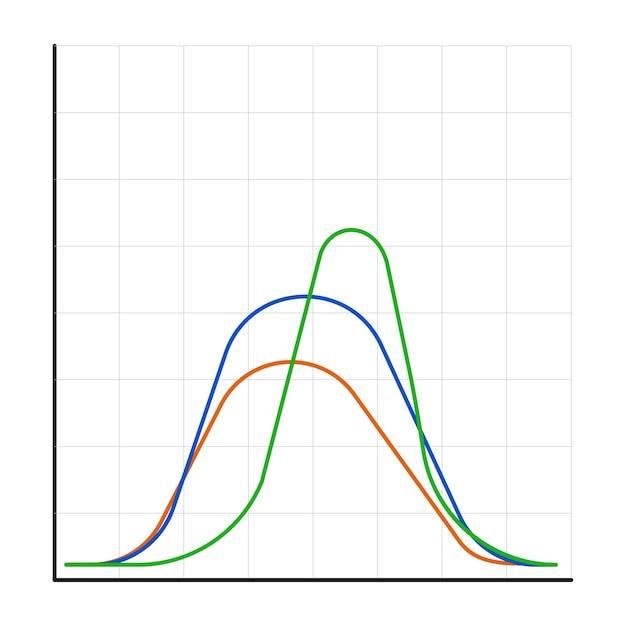
The pH Sweet Spot
Like Goldilocks searching for the perfect porridge, enzymes have a pH sweet spot that makes them feel just right. It’s different for every enzyme, so don’t go using catch-all pH solutions! Some enzymes prefer the acidic side of life, while others thrive in more alkaline environments.
Maintaining pH Balance
Now that we know how pH affects enzyme activity, it’s time to pretend to be chemists and maintain the pH at the sweet spot. Scientists can use buffers, substances that resist changes in pH, to keep the environment just right for the enzyme. It’s like hiring a bouncer to make sure only the right pH molecules get past the velvet rope.
pH and Everyday Life
pH and enzymes don’t just hang out in test tubes; they’re everywhere! In our stomachs, for example, enzymes work their magic to digest our food. The stomach pumps out hydrochloric acid, creating an acidic pH that helps enzymes in the digestion process. So the next time you enjoy a delicious meal, raise a glass to pH and enzymes for their unsung superhero work.
pH may seem like just another acronym, but its influence on enzyme activity is a rollercoaster ride worth taking. By understanding and manipulating pH, scientists can uncover the secrets of enzymes and keep those biochemical parties going strong. So let’s toast to the power of pH, the unsung hero behind the scenes of enzyme activity experiments. Cheers!
FAQ: How does pH affect enzyme activity in experiments?
Welcome to our FAQ section on how pH affects enzyme activity in experiments! Here, we’ll address some commonly asked questions that will help you understand the fascinating relationship between pH and enzyme function. So, let’s dive in!
How do you denature enzymes? ⚗️
When it comes to killing enzymes, we have a fancy word for it – denaturation! Enzymes are delicate little beings, and if you expose them to harsh conditions like extreme heat or strong acids or bases, they lose their shape. And guess what? Without the right shape, enzymes just can’t do their job effectively. So, be nice to your enzymes and avoid subjecting them to such brutality!
How does pH monkey around with enzyme activity? 🙉
Ah, the pH and enzyme saga! pH plays a crucial role in regulating enzyme activity. Each enzyme has its favorite pH range where it performs at its best, known as the optimum pH. When the pH goes above or below this sweet spot, things start to go haywire. The affinity between the enzyme and its substrate weakens, causing a significant drop in its activity. It’s like trying to dance the tango without a partner—it just doesn’t work!
Why is the optimum pH of amylase 7? 🥯
Amylase, the enzyme responsible for breaking down carbohydrates, prefers to dance the night away at pH 7. Why? Well, most biological systems tend to operate around neutral pH, and amylase is no exception. It has evolved to function optimally at this pH because it fits perfectly in the ecosystem of our bodies. It’s like finding the right rhythm on the dance floor—pure harmony!
What is the perfect pH for amylase to show off its moves? 💃
The amylase enzyme throws its best dance party at pH 7. So, if you’re doing an experiment involving amylase, make sure the pH is at this magic number. Otherwise, poor amylase will end up feeling like a wallflower, unable to strut its stuff. Remember, pH 7 is the key to unlocking amylase’s dance moves!
At what pH does the enzyme get its groove on? 🎵
When an enzyme is at the top of its game, we say it has hit the jackpot—you know, found its groove! For most enzymes, including those amylase crowd-pleasers, pH 7 is where the magic happens. It’s like having the perfect playlist that gets everyone on their feet, ready to show off their best moves. So, pH 7 is the place to be for enzymes to have a rocking good time!
What happens when you throw more substrate into the enzyme party? 🎉
Ah, the party gets wild when you add more substrates into the mix! Picture this: you’ve got an enzyme hitting the dance floor, surrounded by substrates—the molecules it wants to work its magic on. When you add more substrates, the enzyme grabs them eagerly, increasing the rate of reaction. It’s like throwing more partygoers onto the dance floor—the rhythm intensifies, and the fun just multiplies!
What if an enzyme gets too drunk? 🍻
Well, if an enzyme gets a little too tipsy, it might end up crashing the entire party. Enzyme inhibition is like the hangover from last night’s shenanigans. Something comes along—a sneaky molecule, known as an inhibitor, trying to sabotage the enzyme’s fun. It messes with the enzyme’s shape, rendering it incapable of performing its usual dance moves. It’s like a dance partner who suddenly loses all coordination—things get awkward real quick!
What happens when enzymes are not in the mood for your pH preferences? 😒
If an enzyme is not in its optimal pH environment, it starts sulking—and trust us, you won’t like it when enzymes sulk! It may look perfectly fine, but its activity takes a nose-dive. Just like when you’re forced to listen to that annoying high-pitched sound that ruins your vibe, an enzyme out of its pH comfort zone loses its groove. So, keep your enzymes happy, and they’ll dance like nobody’s watching!
And that wraps up our pH and enzyme activity FAQ session! We hope you’ve found the answers you were looking for and gained a deeper understanding of this enchanting relationship. Just remember, enzymes are like dance partners—you need to set the right pH and groove to witness their true brilliance!
